International Certificate of Vaccination or Prophylaxis (ICVP)
Information relating to use of the ICVP for yellow fever vaccination

Note: Currently, under International Health Regulations (2005) (IHR), yellow fever (YF) and poliomyelitis are the only vaccinations that can be recorded in an International Certificate of Vaccination or Prophylaxis (ICVP).
The information that follows relates to use of the ICVP for YF vaccination.
Ordering
Yellow Fever Vaccination Centres (YFVCs) in England, Wales and Northern Ireland will receive a corresponding number of ICVPs with each order of YF vaccine placed with Sanofi Pasteur. The current ICVP version (2019) has a template for a Medical Letter of Exemption.
With effect from Friday 26th January 2024, Harlow Printing Ltd will be taking orders for extra supplies of the International Certificate of Vaccination or Prophylaxis (ICVP). Please visit their online shop.
ICVP can also be ordered via their dedicated customer telephone line on 0191 4556901 or 0191 4554286. Lines are open from Monday to Friday 08:30 to 16:30 hours (excluding Public Holidays). Please note, only card payments are accepted.
Price per pack of 10 (minimum 1 pack - maximum 30 packs): £10.00 (inc. VAT at 20% and postage and packaging).
Alternatively, purchase orders can be sent for invoicing (minimum order of 5 packs of 10) to: contracts@harlowprinting.co.uk or either of the account managers:
- Clare Mitchell (clarem@harlowprinting.co.uk)
- Nicci Dickinson (niccid@harlowprinting.co.uk)
In Scotland, YFVCs receive a corresponding number ICVPs with each order of YF vaccine placed with Sanofi Pasteur. Additional copies of the ICVP can be ordered from Public Health Scotland online or by calling the Travel Health Section (yellow fever) on 0141 300 1137.
Completing the ICVP
Although we are certain that most health professionals at Yellow Fever Vaccination Centres complete the International Certificate of Vaccination or Prophylaxis (ICVP) as required, from time to time, we are alerted to examples of poorly completed certificates.
The World Health Organization (WHO), International Health Regulations, are very clear on how the ICVP should be written and state that any amendment, erasure or failure to complete any part of the certificate may render the certificate invalid (Annex 6, vaccination, prophylaxis and related certificates).
For the owner of the certificate, poor documentation may be an issue. We are aware of anecdotal reports of the ICVP being checked at point of entry to a country and challenged for not meeting the WHO standard. The consequences of such a challenge may be serious and could, in a worst-case scenario, include forced vaccination or payment of a fine. The replacement of a poorly documented ICVP prior to travel may also be costly to the traveller and take up valuable clinic time.
The traveller who carries a correctly documented ICVP will be less likely to encounter problems on entry to a country where there is a certificate requirement, so please take a few minutes to revisit our guidance on how to complete the ICVP correctly. You can find written information and an example certificate in the Yellow Fever Zone and we have produced a short video ‘Getting to grips with the yellow fever certificate’ as an alternative way of presenting some practical advice on the topic.
Please do email our clinical team if you would like to bring to our attention any significant issue relating to completing of ICVPs. Whilst we cannot respond to all reports personally, we will follow up reports of serious concern. Your input helps to inform our YF training content and the resources we provide on TravelHealthPro.
The ICVP should be completed in either English or French (and additions to the ICVP may be completed in another language in addition to English or French).
Traveller details
In the spaces provided:
The travellers’ details should be as they appear on their passport.
Citizens of some countries are issued with a national identification (ID) document, and this information can be entered on the ICVP if appropriate. This is not applicable for British nationals and the space for national ID document should be left blank.
The recipient of the vaccine should sign or make a mark for their name. If the recipient is a child, the parent or guardian can sign on the child’s behalf and mark the signature e.g. parent/guardian. Signature does not imply consent, but ownership of the ICVP.
See extra information for transgender travellers.
Vaccine or prophylaxis details
In the spaces provided:
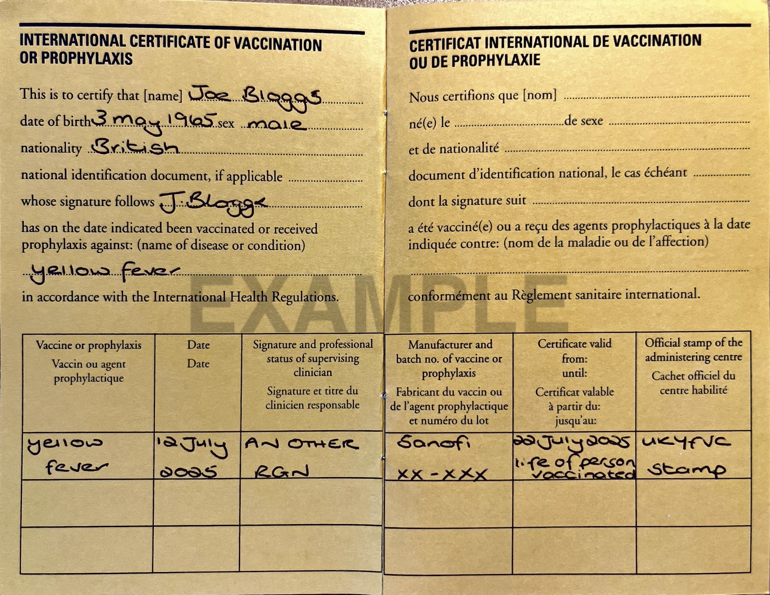
The vaccine or prophylaxis must be stated as e.g. yellow fever.
The date administered must be written as e.g. 1 August 2015 with the month written in full.
Either the Responsible Supervising Clinician (RSC) or other authorised health worker (doctor, nurse or pharmacist) can sign the ICVP.
The manufacturer and batch number of the vaccine should be recorded.
Certificate validity must be stated. When the vaccine is given for the first time, the ICVP becomes valid 10 days from the date of vaccination.
As of 11 July 2016, the ICVP is valid for the 'life of the person vaccinated' [existing ICVP written at any date before 11 July 2016, will be accepted as valid for life, and should not be altered in any way]. The World Health Organization published guidance in 2016 stating that a valid ICVP presented by arriving travellers, cannot be rejected on the grounds that more than ten years have passed since the date vaccination became effective as stated on the certificate and that boosters or revaccination cannot be required.
Country requirements for an ICVP for YF, together with information on certificate validity, are listed on TravelHealthPro Country Information pages.
The official stamp of the administering YFVC should be entered in the space provided.
Example completed ICVP for yellow fever vaccination
Re-issue of ICVP
Replacement of a previously issued ICVP, lost or misplaced or subject to name change
Existing ICVP written to a 10-year validity should be acceptable under International Health Regulations and should not routinely be re-issued (unless subject to name change, see below).
Where the original ICVP has genuinely been lost or misplaced, badly damaged (illegible), or subject to name change (e.g. due to marriage, divorce, gender re-alignment and verified by appropriate documentation) and where an accurate medical record of the previous vaccination can be seen, a doctor, nurse or pharmacist at a Yellow Fever Vaccination Centre (YFVC) can issue a replacement ICVP.
Replacement ICVP must include*:
- Date of original vaccination (NOT the date of reissue)
- Signature and professional status of supervising clinician (i.e. the health professional reissuing the ICVP). Please note, the health professional is signing to confirm information on the replacement ICVP as correct.
- Manufacturer and batch no. of vaccine or prophylaxis (NaTHNaC does not hold current or historical information. Where a batch number alone is documented, it may be possible to identify the manufacturer; where a manufacturer alone is documented identifying a batch number is not possible).
- Official stamp of the administering centre reissuing the ICVP (this may not be the original YFVC). In every case it is important that the health professional reissuing the ICVP is satisfied that the traveller is adequately protected against YF.
- When replacing a genuinely lost or mislaid ICVP, it is acceptable to write the term of validity as valid for the 'life of the person vaccinated’ [WHO personal communication 2017], whatever the date of original issue.
- When replacing a genuinely badly damaged (illegible) ICVP, it is acceptable to write the term of validity as valid for the 'life of the person vaccinated’ whatever the date of original issue [NaTHNaC opinion].
- When replacing an ICVP subject to name change for whatever reason (name must match that on current passport), it is acceptable to write the term of validity as valid for the 'life of the person vaccinated’, whatever the date of original issue [NaTHNaC opinion].
*If details are incomplete or unavailable, revaccination should be considered following the usual risk assessment. Specialist advice should be sought if revaccination is being considered within four weeks of the original vaccination.
We recommend that YFVC purchase additional stock of ICVP to be used where a replacement ICVP is requested (rather than use the ICVP stock that is provided with vaccine orders). Additional ICVP can be purchased from Communisis.
Travellers should be advised to keep their ICVP safe; it is also a good idea for travellers to make a copy (photo or scan) of their certificate as clinical records are not retained permanently.
Getting to grips with the ICVP- a practical guide
NaTHNaC have updated the online guide ‘Getting to grips with the International Certificate of Vaccination or Prophylaxis for yellow fever: a practical guide’. Health professionals are recommended to access this free resource if involved in administering yellow fever vaccinations. Reflecting on this learning activity can contribute towards a health professional's continuing professional development.
*Please note the name of the vaccine manufacturer in the UK has changed from Sanofi Pasteur to Sanofi
Resources
- International Health Regulations (2005). World Health Organization. Geneva.
- World Health Organization Q&A on the extension to life for yellow fever vaccination
- Information page on how to complete the ICVP for polio vaccination
- ICVP (YF or polio) and Hajj/Umrah certificate email enquiry service
- Schönenberger S, Hatz C, Bühler S. Unpredictable checks of yellow fever vaccination certificates upon arrival in Tanzania. Journal of Travel Medicine, Volume 23, Issue 5, 1 September 2016
- Contact Us


